
Obesity in the US is an epidemic impacting over 100 million people in the US and 42% of adults leading to 34 million adults diagnosed with Type 2 diabetes mellitus (T2DM) [1]. Underserved communities are disproportionately impacted, with Black and Latino adults having the highest obesity rates at 49.9 percent and 45.6 percent, respectively. By 2030, nearly half of U.S. adults will be considered obese, including nearly 1 in 4 who will have severe obesity [2]. Most critically, obesity is associated with greater than 200 health complications, including life-threatening heart disease, stroke, and some cancers, leading to an 8-year reduction in life expectancy [3].
While there are multiple treatments available that act by decreasing calorie intake, metabolic adaptation makes long-term weight management through calorie restriction difficult. On top of that, other treatments often show some of the weight loss comes from a loss of lean muscle tissue. We believe there continues to be an unmet need in the treatment of obesity that includes treatments that work by increasing the resting metabolic rate and energy expenditure.
Adipose (fat) tissue comes in two main types: white adipose tissue (WAT), which is the primary site of energy storage, and brown adipose tissues (BAT), which predominantly functions to dissipate energy surplus (lipids) to generate heat, increasing energy expenditure and counteracting obesity [8,9]. The accumulation of the excess, dysfunctional fat (WAT) that characterizes obesity is a major risk factor for many diseases including T2D, cardiovascular disease, hypertension, stroke, arthritis and some types of cancer [10]. However, the presence of brown fat in adult humans is limited, especially in obese subjects.
Existing data strongly suggests that BAT likely plays a significant role in the regulation of body weight, and increasing the amount of this tissue could be a safe and effective therapy to limit obesity [11]. Beige adipocytes which are present in WAT are inducible brown-like cells, which can be converted from white adipocytes to brown. Notably, the density and activity of beige adipocytes correlate with leanness and decreased cardiovascular risk in humans [12,13]. Thus, the transformation of white adipocytes through browning holds great promise as a new therapeutic strategy to raise energy expenditure and reduce adiposity.
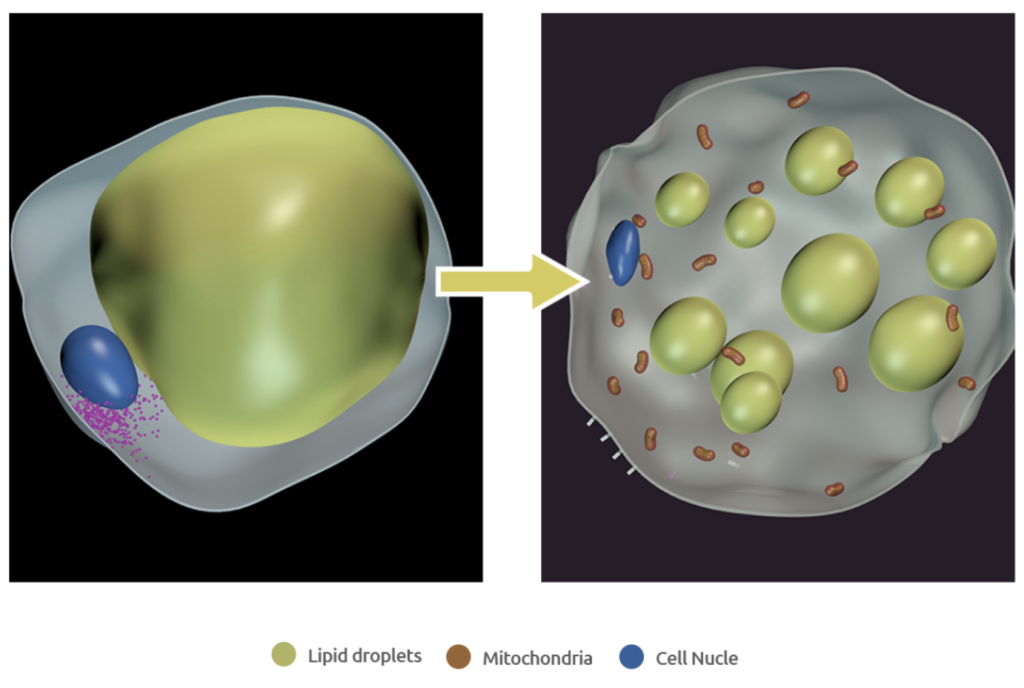

Adipo’s technology uses Notch-inhibiting nanoparticles to convert energy-storing white fat to metabolically beneficial, energy-burning brown fat, leading to weight loss and improved blood glucose and lipid control with no change in calorie intake. Adipo is developing a treatment option that could be used alone or as an orthogonal treatment to enhance the impact of current anti-obesity medicines. Unlike current treatments, Adipo increases energy expenditure with local delivery limiting off-target impacts and side effects. Our hope for the 100+ million people in the U.S., and millions more around the globe, living with obesity is to advance the management of their disease by increasing the calories their body burns.
Adipo’s lead product, ADPO-002NP is composed of proprietary Notch-inhibiting biodegradable poly(lactide-co-glycolide) (PLGA) nanoparticles that are injected directly into adipose tissue, providing localized delivery and sustained intracellular release. Adipo has chosen FDA-approved, biodegradable PLGA polymers which are easily broken down and excreted by the body [4]. The Notch signaling pathway, as a highly conserved intercellular communication mechanism, is a promising regulator of metabolism [5-7]. The locally delivered Notch inhibitor acts intracellularly in the white fat to achieve efficient and sustained Notch inhibition, thereby promoting mitochondrial biogenesis and “browning” of the local white fat.
The proprietary nanoparticle-mediated Notch inhibition therapy is being developed as a weekly, self-administered injection (small 29 gauge needle) to provide the benefits of weight loss and glucose control for people with obesity and T2DM. The physiological effects of the newly formed brown adipose tissue will drive greater energy expenditure and improved insulin sensitivity. Adipo’s innovative therapy combines the recent discovery of the role played by Notch signaling in adipocyte browning, with advances in polymer-based drug delivery, to minimize the risk of systemic toxicity and side effects. Adipo’s treatment offers the potential of a unique value proposition for the treatment of obesity and diabetes, providing the potential for durable weight loss, improved glucose and lipid control and other metabolic benefits while minimizing off-target side effects. Adipo has the potential to play a pivotal and orthogonal role with other medications in the treatment of obesity and diabetes.
To learn more about how our lead candidate ADPO-002NP works to increase brown fat watch the video
Preclinical data for ADPO-002NP have demonstrated promising results in rodents, pigs, and human fat studies. Proof-of-concept animal studies have confirmed inhibition of Notch signaling through local nanoparticle-based delivery induces browning of white adipocytes, leading to weight loss and improved glucose and lipid control [4] [5]. The Adipo therapy has the opportunity to deliver a unique approach to treating obesity and T2DM, as the first treatment to: induce browning; directly transform fat; decrease weight through increased energy expenditure; and lower blood glucose through improved insulin resistance with no change or reduction in caloric intake.
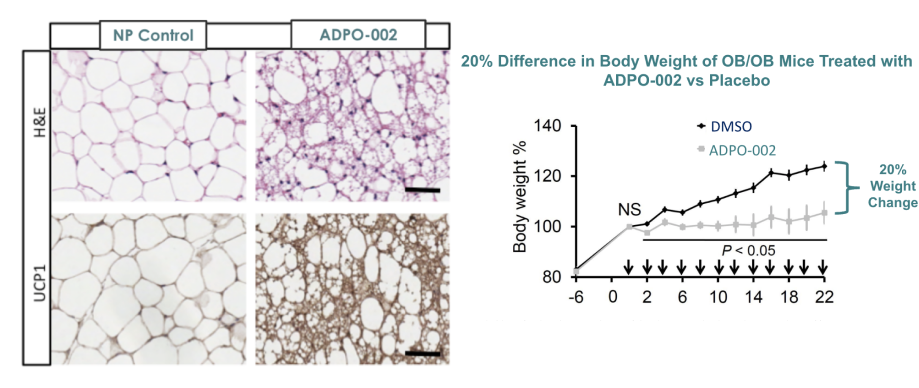
Left graph: C. Jiang et al. Mol Ther. 2017;25(7):1718-1729
Right graph: P. Bi et al. Nat Med. 2014;20(8):911-8
Preclinical studies to date for ADPO-002NP have not shown any safety signals and there is a low potential for off target impacts or side effects due to the limited biodistribution.
Biodistribution studies have shown that both the Notch-inhibitor and the nanoparticle remain proximal to the injection site and have very low biodistribution or systemic circulation in the treated animals. ADPO-002NP is injected locally and is taken up quickly by the white adipose tissue through endocytosis. Within the WAT, Notch-inhibition induces intercellular cascade that results in beiging and the creation of metabolically active adipose tissue. This metabolically active tissue then works systemically to draw excess lipids and glucose from throughout the body to the sites of newly formed brown adipose tissue. As a result of the MOA and lack of systemically circulating drug, there is low potential for off-target side effects.

C. Jiang et al. Mol Ther. 2017;25(7):1718-1729
These data were shared in an oral presentation at the American Diabetes Association 84th Scientific Sessions. ADPO-002 (active ingredient) and ADPO-002 NP (drug product) were studied to determine their impact on human adipose cells and tissue. ADPO-002 was studied to determine its impact on human white adipose tissue harvested from bariatric surgery with patent consent. These studies were conducted to confirm that treatment of human adult fat with ADPO-002 induces browning as demonstrated by the upregulation of two key gene markers PRDM16 and PGC1a which are important in mitochondrial biogenesis and browning of white adipose tissue.


ADPO-002, a Notch inhibitor, promotes the expression of two key browning biomarkers (PRDM16 and PGC1a) in studies using adult human white adipose tissue and human adipose cells. These data support the translation of this novel mechanism of action in humans.
Learn more about our findings and view the ADA oral presentation slides here.
Intellectual Property Technology platform protected by US 15/771,312; US 18/017,384; US 15/621,627; International: PCT/US16/58997; PCT/US21/42867. EU (EP3368553B1) and China (CN108431028B) patents granted.
IP Counsel Ivor Elrifi, JD, Cooley LLP
1. https://www.cdc.gov/obesity/data/adult.html, Adult Obesity Facts
2. Healy Melissa, By 2030, nearly half of all US adults will be obese, experts predict, The Los Angeles Times, December 19, 2019
3. Yuen M., Earle R., Kadambi N., et al. A systematic review and evaluation of current evidence reveals 236 Obesity-Associated Disorders (OBAD). Massachusetts General Hospital & George Washington University. Poster presentation
4. Huang D, Deng M, Kuang S. Polymeric Carriers for Controlled Drug Delivery in Obesity Treatment. Trends Endocrinol Metab. 2019;30(12):974-89.
5. Bi P, Kuang S. Notch signaling as a novel regulator of metabolism. Trends Endocrinol Metab. 2015;26(5):248-55.
6. Gridley T, Kajimura S, Nat Med. 2014;20(8):811-2.
7. Wong W, Sci Signal. 2014:7(338):ec211.
8. Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444(7121):847-53.
9. Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19(10):1252-63.
10. Bray GA, Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine. 2006;19:109-117.
11. Seale P, Lazar M. Brown fat in humans: turning up the heat on obesity. Diabetes 2009;58(7):1482-1484.
12. Pfannenberg C, Werner MK, Ripkens S, Stef I, Deckert A, Schmadl M, Reimold M, Häring H-U, Claussen CD, Stefan N. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes. 2010;59(7):1789-93.
13. Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier A, Richard D. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab. 2011;96(1):192-9.